
My objective here was merely to help you visualize the “electron” for what it truly is. However, such mathematics seems outside the purview of this piece. The complex world of quantum mechanics mathematically represents the electron cloud as a quantum wave function governed by probabilities. Of course, this has been a very simplified explanation of an electron cloud. A cloud is merely the best way to illustrate the true state of an electron. Despite it being represented by a cloud, it cannot be broken down into partials, as the cloud isn’t physical. You can either possess the whole electron or none of it. When your spoon holds 25% of the electron cloud, your spoon possesses a 25% chance of containing the electron mass of 9.11 X 10 – 31 kg. Does your spoon then contain a weight equal to 25% of 9.11 X 10 – 31 kg ? No. You take that spoon and dip it into 25% of the electron cloud area. What about a part of the cloud? Can a part of the cloud have a mass that is less than the electron? Not really. This begs the question, though, d oes the entire cloud together weigh 9.11 X 10 – 31 kg ? Yes. So, h ow can we ascertain its mass? Does an electron cloud even have mass? Science textbooks everywhere confidently illustrate in big bold fonts that an electron has a mass of 9.11 X 10 – 31 kg. In other words, the radius of the electron cloud or the radius of maximum probability is 0.529 Å.W e’ve established that an electron isn’t a perfect sphere revolving around the nucleus, but rather a dense cloudy region of probability. But according to the wave mechanical or cloud concept model, the electron keeps on moving away or towards the nucleus and the maximum probability of locating it lies at a distance of 0.529 Å from the nucleus. This distance is called the Bohr radius and is approximately 0.529 Å ( 0.529 ×10 −10 m). The electrons were like a blur which is where they got the 'Electron Cloud' from.Īccording to Bohr's calculations for a hydrogen atom, the electron under normal conditions always stays at a certain distance from the nucleus. The atoms were traveling at such a high speed that there was no predictable time or place for the electron to be. The electron cloud model is currently the accepted model of an atom. The model is a way to help visualize the most probable position of electrons in an atom. The electron cloud model was developed in 1926 by Erwin Schrödinger and Werner Heisenberg.
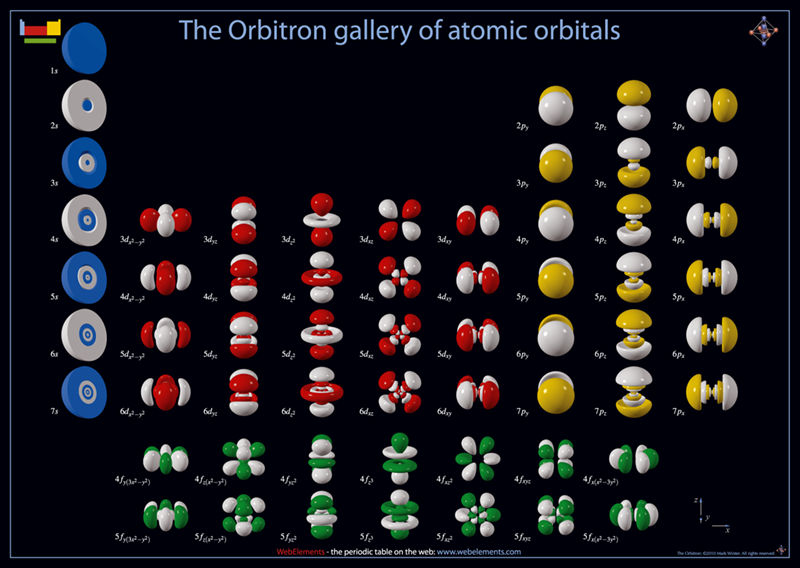
Atomic orbitals also explain the patterns in the periodic table. Using quantum mechanics, chemists can use the electron cloud model to assign electrons to different atomic orbitals. This can be proven by the repeating patterns of chemical properties in the periodic table. the s,p,d,f orbitals are all shaped differently. The sub-orbitals s,p,d,f, are regions where it will be more likely to find electrons, and can each hold a different number of electrons. The shells, k,l,m,n,o,p,q, each represent different levels of energy, and are also called energy levels.
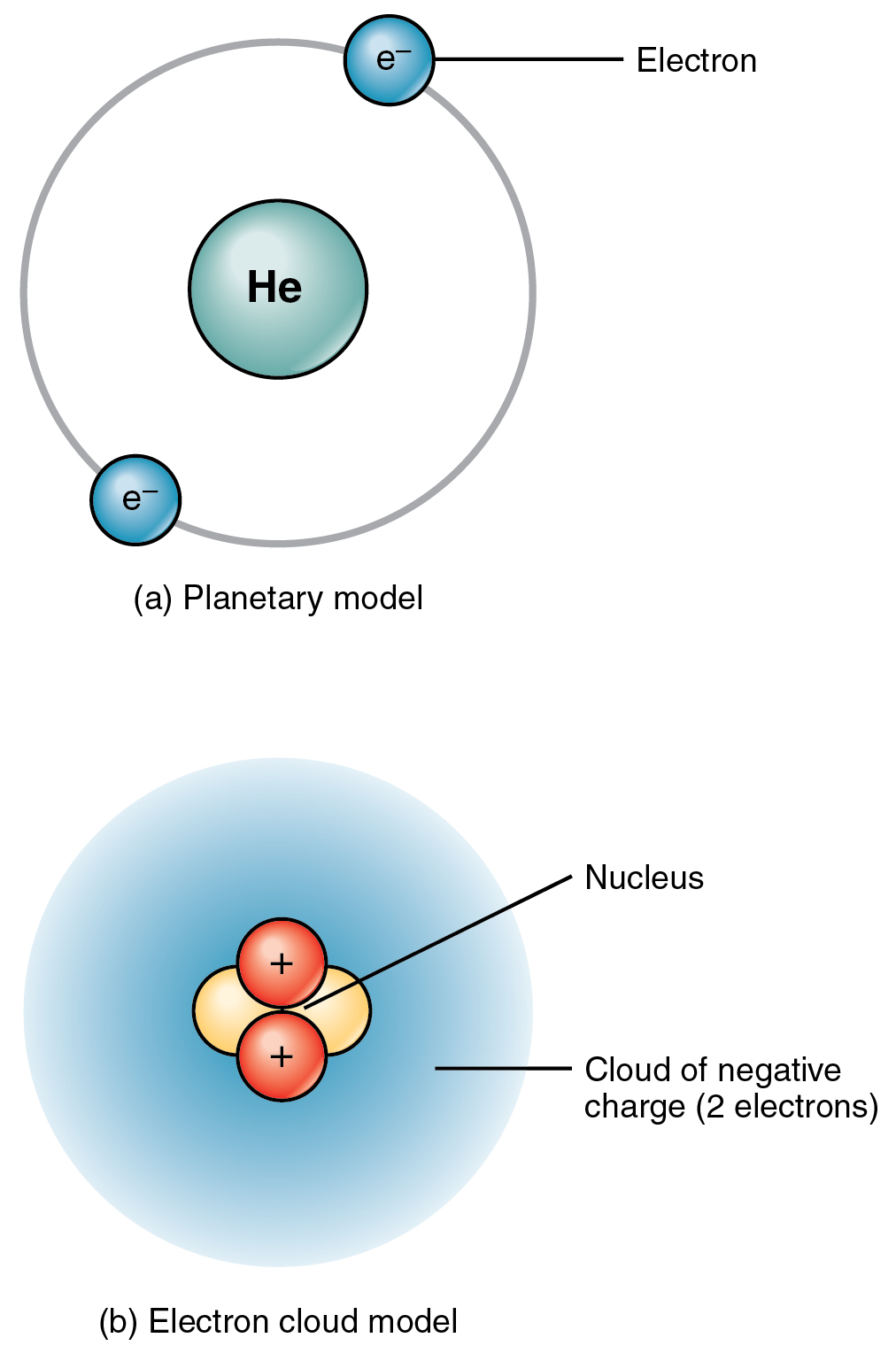
In the Bohr model, electrons are assigned to different shells. The orbitals are specified by shells and sub-orbitals. The electron cloud model says that we cannot know exactly where an electron is at any given time, but the electrons are more likely to be in specific areas. Explaining the behavior of these electron "orbits" was a key issue in the development of quantum mechanics. Bohr talked about electrons orbiting the nucleus.
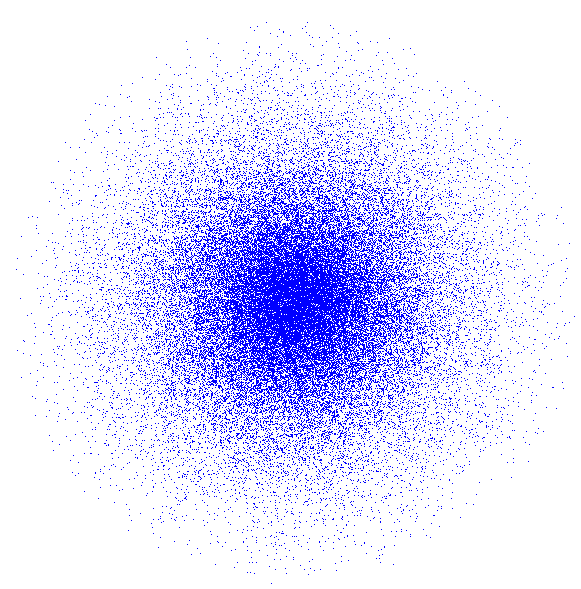
An electron cloud model is different from the older Bohr atomic model by Niels Bohr. The electron cloud is not really a thing. Electron cloud is an informal way to describe an atomic orbital.


 0 kommentar(er)
0 kommentar(er)
